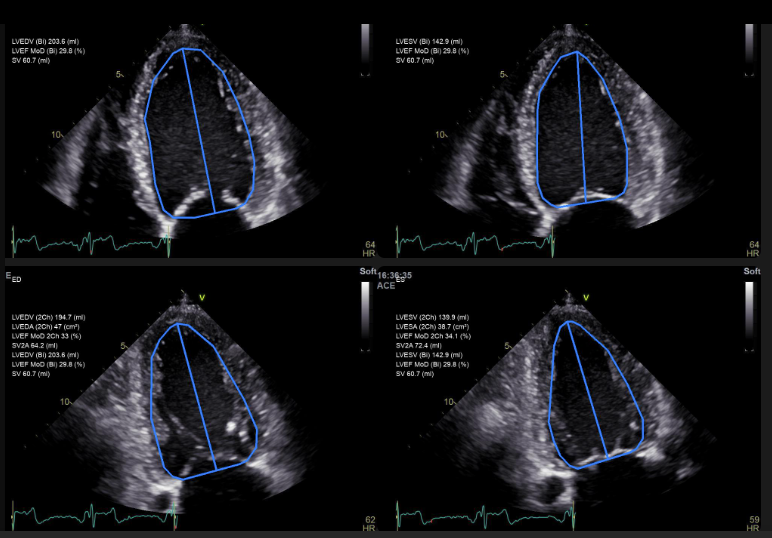
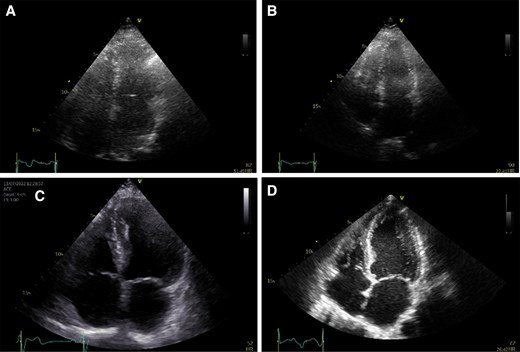
Ligence Heart analysis and reporting system for transthoracic echocardiography has been certified under MDR 2017/745.
The device is regulated as medical device class IIa under the framework of the European Commission.
The Ligence Heart AI system will be now offered to care centres in Europe to aid with the burdens of the echocardiography procedure.
The company expects first sales to happen in Lithuania and the Baltic region, followed by the Netherlands.